Microbiology
Our Products
We Provide Various Solutions
- Highlights
- Features
- More Information
The MALDI Biotyper® sirius System: unveiling new horizons in microbial analysis
Enhanced capabilities
The Bruker MALDI Biotyper sirius System harnesses the cutting-edge technology that has made its predecessors so highly regarded. Equipped with a high-capacity vacuum system and a rapid smartbeam™ lifetime* laser, this system is primed for exceptional operation.
*Lifetime refers to 7 years or 500 million shots, whichever comes first.
** Negative ion mode is for Research Use Only
Enhanced capabilities
The Bruker MALDI Biotyper sirius System harnesses the cutting-edge technology that has made its predecessors so highly regarded. Equipped with a high-capacity vacuum system and a rapid smartbeam™ lifetime* laser, this system is primed for exceptional operation.
*Lifetime refers to 7 years or 500 million shots, whichever comes first.
** Negative ion mode is for Research Use Only
Positive/Negative Ion Mode: unveiling new dimensions and supporting collaborative progress
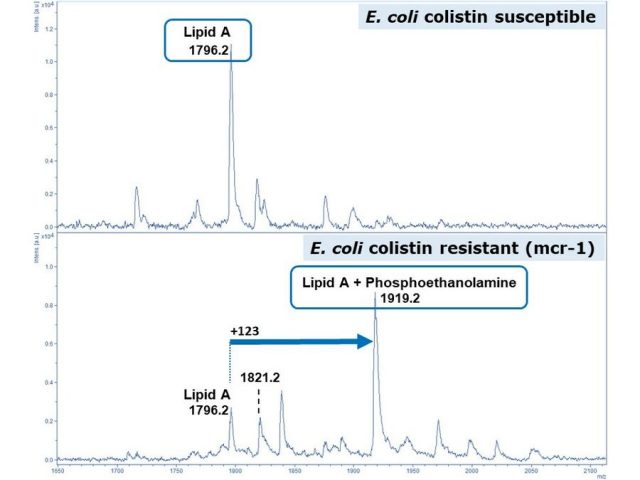
Our revolutionary MALDI Biotyper sirius System is not limited to routine microbial identification. While positive ion mode remains a cornerstone for everyday analysis, the introduction of negative ion mode opens up exciting avenues in microbial research. Dive into cutting-edge lipidomics investigations, uncovering new insights and rapid detection techniques for e.g. resistance-related modifications of lipid A in colistin-resistant bacteria. 1,2
Join the ranks of visionary researchers who are revolutionizing microbial analysis. With the MALDI Biotyper sirius System, you gain access to a world of possibilities.
¹Dortet L et al., 2018, Scientific Reports, Vol: 8, ISSN: 2045-2322
²Dortet L et al., 2018, Journal of Antimicrobial Chemotherapy, Vol: 73, Pages: 3359-3367, ISSN: 0305-7453
MALDI Biotyper sirius IVD System
An IVD CE system for in vitro diagnostic identification of microorganisms originating from human specimens.
For professional use only. Not for sale in the USA.
- MALDI Biotyper Clinical, IVD-CE (Brochure) (PDF, 9 MB)
- MBT Compass HT, IVD-CE (Flyer) (PDF, 282 KB)
- Expert Insights (MALDI Mass Spectrometry Advances Biomedical Clinical Applications) (PDF, 804 KB)
MALDI Biotyper sirius CA System
Cleared USA FDA approval under Section 510(k) for the identification of gram-negative and gram-positive bacteria, anaerobic bacteria and yeast, cultured from human specimens.
Only for sale in the USA and Puerto Rico. For prescription use only.
- MALDI Biotyper CA System (Brochure) (PDF, 4 MB)
- Expert Insights - Advancing Infectious Disease Testing with MALDI-TOF Mass Spectrometry (PDF, 302 KB)
- Expert Insights - Advanced Microbial Identification with MALDI Mass Spectrometry for Streamlined Therapeutic Treatment (PDF, 551 KB)
MALDI Biotyper sirius RUO System
For microorganism identification in research applications.
For Research use only. Not for use in clinical diagnostic procedures.
- MALDI Biotyper RUO/GP (Brochure) (PDF, 4 MB)
- MBT Compass HT, GP/RUO (Flyer) (PDF, 280 KB)
- Expert Insights - MALDI Biotyper® advances the development of clinical microbiology (PDF, 423 KB)
MALDI Biotyper sirius GP System
For industrial applications such as Food, Veterinary, Water or Pharma environments.
Not for use in clinical diagnostic procedures.